
Prostate Cancer Diagnostics

Working on Solving Prostate Cancer
Cutting Edge Healthcare Technologies

Prostate Cancer
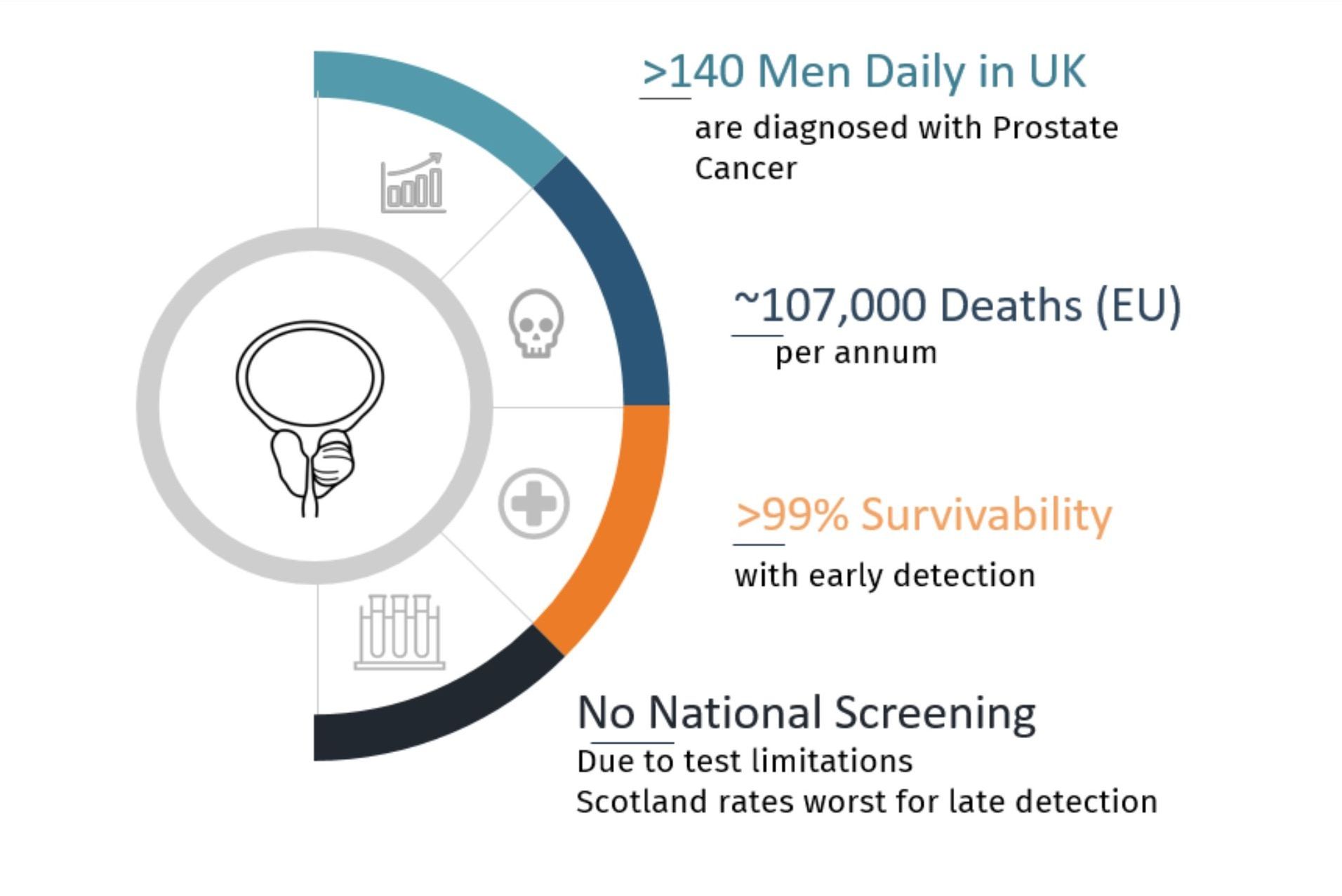
Prostate cancer is the most common cancer diagnosed in males in the UK, with over 56,000 new cases in 2020 and projected to reach over 75,000 in 2040. Yet the survival rate of the cancer is high in the first 3 stages of cancer (99%). It only drops as the cancer progresses to stage 4, indicating the importance of early diagnosis and continuous surveillance. With the increase of mortality and incidents, the market for prostate cancer screening is likely to rise rapidly. In the UK alone, 12,000 men die from prostate cancer each year, and 1 in 8 UK men will be diagnosed with prostate cancer in their lifetime. Alarmingly, in Scotland, the percentage of men diagnosed too late for a cure is more than double that of other parts of the UK (Prostate Cancer UK).
What We Do

Biosensing is What We Do
At Pinpoint Medical, we are working on solving prostate cancer screening through detection of novel biomarkers with our proprietary chemical sensing technology.
We are also developing our intelligent Air Quality (iAQ) platform that can clean the air of infectious pathogens and automatically test our environment for multiple disease related pathogens.

1 in 8 men are diagnosed with prostate cancer. 35% are too late for cure.
Handheld Urine Test
Pinpoint Medicals prostate cancer diagnostics will allow regular screening at the primary care stage with a 15min automated handheld urine test. This would allow improved early detection and survivability.

Prostate Cancer
Prostate cancer is the most common cancer diagnosed in males in the UK, with over 56,000 new cases in 2020 and projected to reach over 75,000 in 2040. Yet the survival rate of the cancer is high in the first 3 stages of cancer (99%). It only drops as the cancer progresses to stage 4, indicating the importance of early diagnosis and continuous surveillance. With the increase of mortality and incidents, the market for prostate cancer screening is likely to rise rapidly. In the UK alone, 12,000 men die from prostate cancer each year, and 1 in 8 UK men will be diagnosed with prostate cancer in their lifetime. Alarmingly, in Scotland, the percentage of men diagnosed too late for a cure is more than double that of other parts of the UK (Prostate Cancer UK).
What We Do

1 in 8 men are diagnosed with prostate cancer. 35% are too late for cure.

Biosensing is What We Do
At Pinpoint Medical, we are working on solving prostate cancer screening through detection of novel biomarkers with our proprietary chemical sensing technology.
We are also developing our intelligent Air Quality (iAQ) platform that can clean the air of infectious pathogens and automatically test our environment for multiple disease related pathogens.

Pinpoint Medicals prostate cancer diagnostics will allow regular screening at the primary care stage with a 15min automated handheld urine test. This would allow improved early detection and survivability.

Prostate Cancer
Prostate cancer is the most common cancer diagnosed in males in the UK, with over 56,000 new cases in 2020 and projected to reach over 75,000 in 2040. Yet the survival rate of the cancer is high in the first 3 stages of cancer (99%). It only drops as the cancer progresses to stage 4, indicating the importance of early diagnosis and continuous surveillance. With the increase of mortality and incidents, the market for prostate cancer screening is likely to rise rapidly. In the UK alone, 12,000 men die from prostate cancer each year, and 1 in 8 UK men will be diagnosed with prostate cancer in their lifetime. Alarmingly, in Scotland, the percentage of men diagnosed too late for a cure is more than double that of other parts of the UK (Prostate Cancer UK).
What We Do
1 in 8 men are diagnosed with prostate cancer. 35% are too late for cure.

Biosensing is What we Do
At Pinpoint Medical, we are working on solving prostate cancer screening through detection of novel biomarkers with our proprietary chemical sensing technology.
We are also developing our intelligent Air Quality (iAQ) platform that can clean the air of infectious pathogens and automatically test our environment for multiple disease related pathogens.
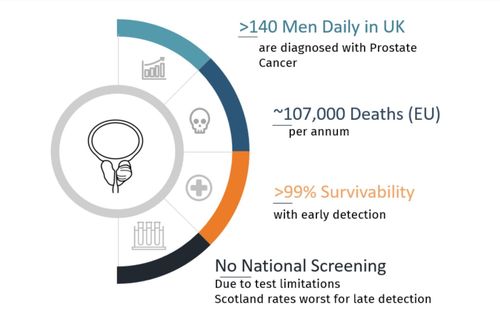
Pinpoint Medicals prostate cancer diagnostics will allow regular screening at the primary care stage with a 15min automated handheld urine test. This would allow improved early detection and survivability.
iAQ intelligent Air Quality

iAQ intelligent Air Quality
A single bioaerosol droplet released from our breath can have over 1 million covid viruses.
Bioaerosols are a major source of disease transmission
Hospital acquired infections lead to >300,000 additional infections
It is costing NHS England >£2 billion each year
Pinpoint Medicals iAQ
Pinpoint Medicals iAQ is a fully modular system capable of functioning in ventilation systems or stand alone in wards and closed public spaces.
The iAQ will provide a healthy environment monitored
continuously to maintain standards.
iAQ intelligent Air Quality

iAQ intelligent Air Quality
A single bioaerosol droplet released from our breath can have over 1 million covid viruses.
Bioaerosols are a major source of disease transmission
Hospital acquired infections lead to >300,000 additional infections
It is costing NHS England >£2 billion each year
Pinpoint Medicals iAQ
Pinpoint Medical's iAQ is a next-generation, fully modular air quality solution—engineered to integrate seamlessly into ventilation systems or function independently in any environment, from clinical wards to high-traffic public spaces.
iAQ safeguards air quality—24/7 monitoring, zero compromise..

iAQ intelligent Air Quality

Pinpoint Medical's iAQ is a next-generation, fully modular air quality solution—engineered to integrate seamlessly into ventilation systems or function independently in any environment, from clinical wards to high-traffic public spaces.
iAQ safeguards air quality—24/7 monitoring, zero compromise..
Smart Innovation. Real Impact.
Advanced Diagnostics & Environmental Monitoring
Effortless Operation
Designed for simplicity: easy to deploy, intuitive to use, and backed by clear, actionable data that helps teams respond quickly and maintain high standards.
Cost-Efficient by Design
Streamline the diagnostics cycle with continuous monitoring and automated reporting—reducing manual checks, downtime, and unnecessary expenditure.
Assured Quality, Every Time
Consistent, trackable diagnostics with built-in indicators for early intervention. Documentation and reporting made simple to support compliance and continuous improvement
Advanced Diagnostics & Environmental Monitoring
Effortless Operation
Designed for simplicity: easy to deploy, intuitive to use, and backed by clear, actionable data that helps teams respond quickly and maintain high standards.
Cost-Efficient by Design
Streamline the diagnostics cycle with continuous monitoring and automated reporting—reducing manual checks, downtime, and unnecessary expenditure.
Assured Quality, Every Time
Consistent, trackable diagnostics with built-in indicators for early intervention. Documentation and reporting made simple to support compliance and continuous improvement
At Pinpoint Medical, we believe prevention starts with visibility.
Our
iAQ system continuously monitors bioaerosol levels and airborne pathogens in real time—giving healthcare teams the power to act before infection spreads.
Modular, intelligent, and built for precision, iAQ helps create safer spaces, reduce transmission risk, and drive down the cost of preventable infections.
Because when you can see the invisible, you can stop it.
At Pinpoint Medical, we believe prevention starts with visibility.
Our
iAQ system continuously monitors bioaerosol levels and airborne pathogens in real time—giving healthcare teams the power to act before infection spreads.
Modular, intelligent, and built for precision, iAQ helps create safer spaces, reduce transmission risk, and drive down the cost of preventable infections.
Because when you can see the invisible, you can stop it.

Welcome
Jason Donnelly - CEO, Pinpoint Medical
"At Pinpoint Medical, innovation and precision are at the heart of everything we do. We're pioneering next-generation biosensing and pathogen detection technologies to transform diagnostics, infection control, and environmental safety.
As you explore our site, you'll discover how our advanced systems are redefining real-time monitoring and empowering proactive healthcare.
Thank you for visiting — we’re excited to share our vision and shape the future of medical technology, together."

Welcome
Jason Donnelly - CEO, Pinpoint Medical
"At Pinpoint Medical, innovation and precision are at the heart of everything we do. We're pioneering next-generation biosensing and pathogen detection technologies to transform diagnostics, infection control, and environmental safety.
As you explore our site, you'll discover how our advanced systems are redefining real-time monitoring and empowering proactive healthcare.
Thank you for visiting — we’re excited to share our vision and shape the future of medical technology, together."
